Fusion QbD® from S-Matrix®
Fusion QbD Software Platform - Lifecycle Management Software for the Development, Validation, and Transfer of Analytical Methods
Fusion QbD is a modular software platform providing bidirectional data exchange to various chromtography systems and complete CFR21 Part11 support.
DoE - An Indispensable QbD Tool
The application of QbD principles using DoE is already common in product development ICH Q8(R2) and now also described for analytical development as an extended approach in ICH Q14. With Fusion QbD those principles can easily be applied one-to-one to the development of chromatographic methods.
Traditionally, LC methods are developed according to an OFAT (One Factor At a Time) approach. This approach is not only time consuming, but also suppresses the effect of interactions between the relevant qualitative and quantitative study factors. Only by examining all levels for all factors simultaneously, we can gain comprehensive knowledge of the correlations in total.
What is the Difference between DoE and Theoretic Chromatography Models?
Design of experiments (DoE, german: Statistische Versuchsplanung) is a method for planning experiments that assumes that all experimental results are overlaid by unavoidable random errors. The random errors are taken into account using statistical methods not only during the evaluation, but also during the experimental planning, i.e. before the start of the experimental design phase.
Design of experiments (DoE) pursues several goals:
- DoE is designed to obtain maximum information with minimal experimentation. In short: You only want to carry out as many experiments as necessary to obtain all information you need. Not too many experiments, but not too few either.
- Unlimited number of study factors (Critical Method Parameters, CMP) included in a single study
- Unlimited number of process characteristics (Critical Method Attributes, CMA) included in a single study
- Precise characterization of interactions between all study factors included in the same study
Fusion QbD is the only software designed for the development of chromatographic methods that uses the real and complete QbD package aligned with ICH Q8(R2). All predictions are based on empirical models generated from experimental raw data, not theoretic “first principle” functions or in-house algorithms. This allows Fusion QbD to be applied to all separation methods: Chiral, HILIC, Ion Exchange, Size Exclusion, Normal Phase and, of course, Reverse Phase. Fusion QbD is also powerful with difficult sample matrix where baseline separation is not achieved.
DoE for Everybody
Learning experimental design and data modelling theories usually is a challenging task. However, without substantial expertise, conventional statistical software cannot be used effeciently and mistakes are inevitable. In everyday business life, however, it difficult to obtain the required depth in expertise on one’s own and the software can’t be used without the support of an experienced statistician.
Fusion QbD provides a technology by which even the statistical layman can easily create and evaluate DoE experiments.
For this purpose S-Matrix has developed unique automation algorithms, that have given evidence to provide the same satisfactory outcome as in the user-interactive mode. Therefore, the user-interactive mode in Fusion QbD is only required, if one wants to force the software to create specific designs or specific models
Software Demo and Trial Phase
Please contact us if you would like to have the software presented. We would be happy to discuss your questions and requirement and can also provide you with the software for an assisted trial phase.
Fusion Method Development (FMD)
Chemistry Screening
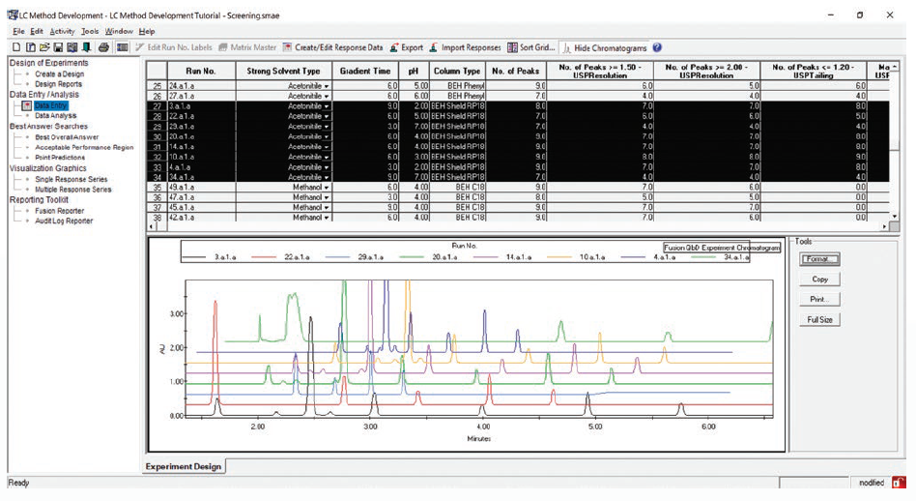
Fast, Automated Screening of Multiple Column and Mobile Phase Chemistries
Supports All Separation Modes – e.g. RPC / NPC / SEC / IEX / HILIC / Chiral / SFC
PeakTracker™- Automated PDA & MS Spectra Based Peak Tracking
Automates, optimizes and simplifies the use of PDA and MS data in LC and LC/MS method development.
Complex challenges PeakTracker can address include:
- Auto-deconvolution of co-eluted peaks
- two or more peaks with identical mass
- non-ionizing & non-absorbing compounds
Optimization with Integrated Robustness
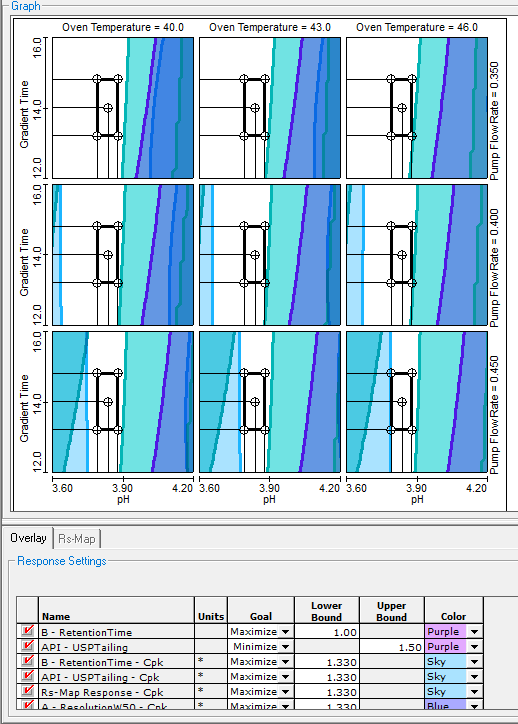
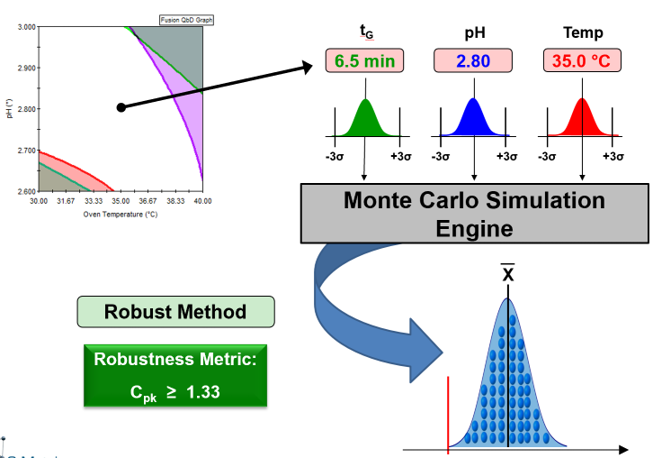
- Fully Characterizes Robustness Throughout Entire Experimental Region
- Establishes Robust Method Operable Design Region (MODR)
- Simultaneously Address All Your Suitability Requirements
- Regulatory Accepted Robustness Metrics – e.g. Cp, Cpk, Cpm, % RSD, …
Forced Degradation Study Samples
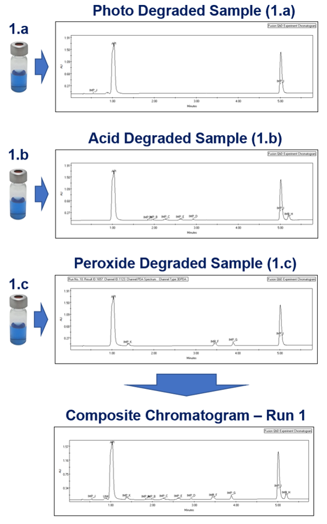
- Full Automation Support
- Peak Tracking Across Deg. Samples
- Composite Chromatogram Modeling and Visualization
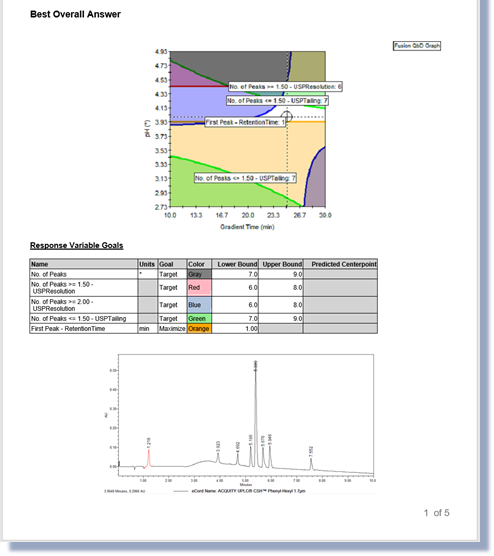
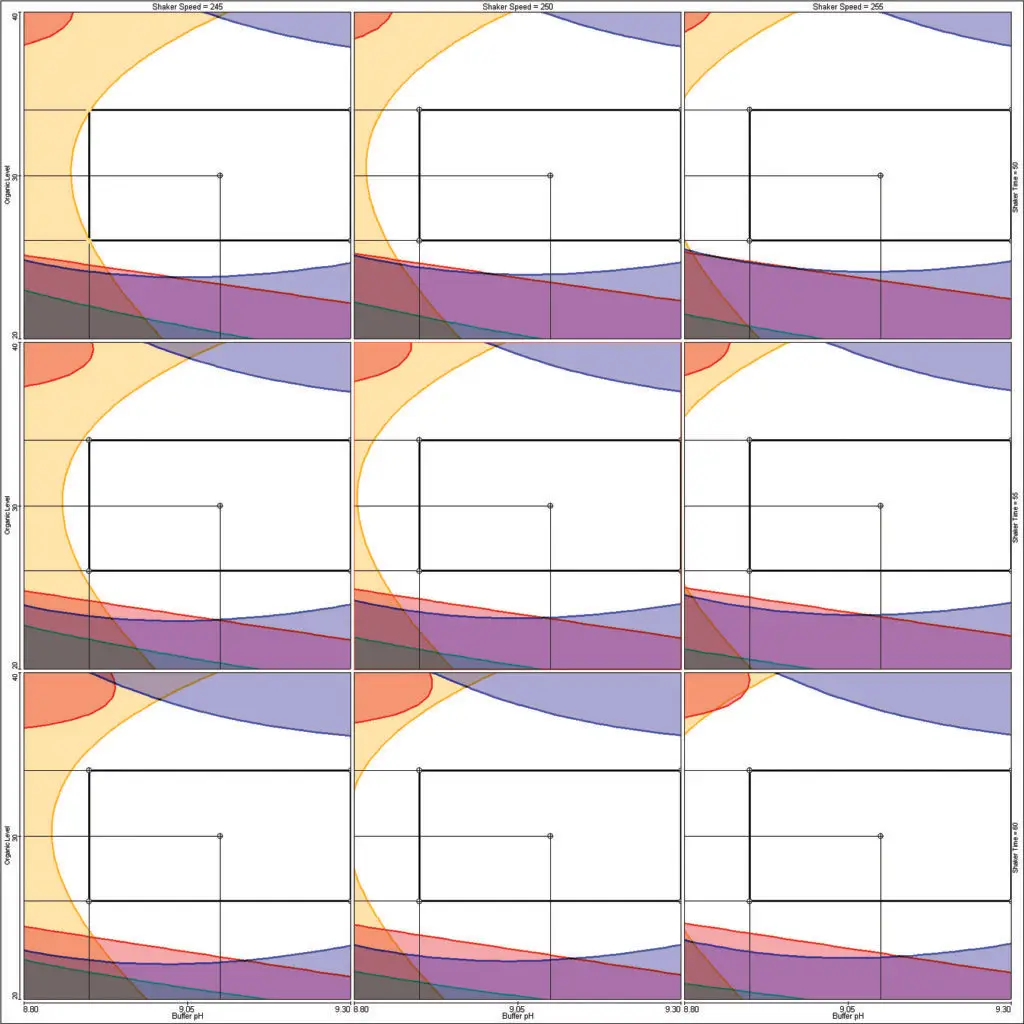
Resolution Map Response

A single peak response for the least resolved peak pair
- Graduated heat map in a 2D contour graph or 3D response surface graph:
Helps to identify regions with stable peak resolution.
- Combination with other responses in a traditional Fusion QbD overlay graph:
Helps to identify regions where all method goals are met simultaneously.
Fusion Process Development (FPD)
Quality by Design on a Plate!
If you are wondering where to start when implementing Quality by Design in your organization, Fusion Product Development (FPD) is the answer you’ve been looking for. With automated experimental design selection, validated data exchange, sophisticated data analysis tools, and comprehensive reporting, FPD is the perfect place to start your QbD journey.
Non-LC Method Development
Fusion QbD works best for LC methods, as we can provide full automation support for supported LC instruments. Still, with FPD any non-LC method (e.g. GC, CE) can be studied. Although instrument support is not given, data exchange with your CDS is still possible.
Thus, you can as well export your study protocol as a sequence into your CDS and import processed peak results back to Fusion QbD. However, the main restriction is that the methods for each experimental run still need to be created in your CDS manually, before the sequence is ready for data acquisition.
Sample Prep Studies
As any study factor can be defined in FPD, this module is applicable for any process. Therefore, FPD can be a welcome addition to your chromatographic studies, if you want to study the sample preparation procedure, as well.
Fusion Method Validation (FMV)
Full Method Validation Experiment Suite
- Calculations and reporting meet all current FDA/ICH/USP validation guidances – including the new USP‹1210›!
- Can be used for LC and Non-LC methods (e.g. GC, CE, Q-NMR)!
- Automates LC method validation experiments on multiple instruments and CDS systems!
- Regulatory accepted validation for both Small & Large Molecules!
- Statistically rigorous and defensible robustness testing!
- Handles multiple compounds – creates complete reports for each!
- Shortens your LC method validation time by as much as 75%
Fusion Inhaler Testing (FIT)
The Only fully Automated Off-the-Shelf Software Solution for In-Vitro Inhaler Testing!
- Can be used for all DUSA and Inhaler Testing work – ACI, MMI, MSLI, FSA, FSI, and NGI ®!
- Automates LC work on multiple instruments and CDS systems!
- Part 11 support for regulatory compliance!
- Built-in workflow management system with secure templating!
- Reduces or eliminates labor intensive manual data and report review!
- Reduces analyst time by a minimum of 40%!
Training Concept
How to get your employees qualified in the operation of Fusion QbD?
We as a vendor of the Fusion QbD software have a high interest that your employees learn how to use the software correctly. For that purpose your users need get trained in the Fusion QbD technologies and in the operation of the software.
Only with the right understanding of the different technologies that are applied in Fusion QbD a correct software operation is possible. We also know, that the correct operation of Fusion will be a process driven by experience.
Accounting for the above, we as a vendor provide the following services and solutions to support our customers in getting their employees trained and qualified:
- Fusion QbD Training Program: Basic education in the applied technologies and the use of the software based on our training data set.
- Fusion QbD Public Webinars
- Advanced Training: Customized training for experienced user based on user-defined topics
- User Guides: Detailed description of workflows and explanation of single features and functions.
- Customer Support as part of the Fusion QbD Support & Maintenance Agreement: E.g. assistance in the use of single features, setting up designs, explanation of new features implemented with a release update.
- Remote Consultancy Services based in individual agreements, e.g. working together on a real method development project
Although we provide a basic protocol of how data need to be generated and prepared in the connected chromatography data system, the correct operation and interpretation of the CDS data is out of scope from our services and we need to expect that a Fusion QbD user is familiar with the connected CDS as an average experienced chromatographer in order to provide data of good quality for import to Fusion QbD.
However, as Cromingo also provides expert know how for Waters Empower™, we can help out here as well. Please check out our section for CDS Services.
Additional Services
Certified Software Qualification
In cooperation with S-Matrix, two Fusion QbD software qualification options are offered:
Independent qualification: The software qualification is performed by the customer with the S-Matrix QMD package (see below).
On-site software qualification. Software qualification is performed by an S-Matrix certified representative on site or remotely.
Qualification Master Documentation Package (QMD Package)
We will provide a Software Qualification Master Documentation Package (QMD Package) for each release version of Fusion QbD. The QMD contains all documents, testing plans, and data sets required to fully qualify Fusion QbD software installation and operation in your environment. The QMD covers:
- All 21 CFR Part 11 compliance support tools and functions.
- All installed Fusion Application Modules (FAMs – e.g. Fusion LC Method Development, Fusion LC Method Validation)
- All CDS Data Exchange Modules (DEMs) – e.g.:
- » Agilent ChemStation/OpenLab DEM
- » Thermo Scientific Chromeleon DEM
- » Waters Empower DEM
We also maintain a complete documentation set to support customer software qualification:
- 21 CFR 11 Support Assessment Report
- GAMP 5 Classification Document
- Risk Assessment Document
- Deployment Diagrams
- Detailed Installation/Setup Guides
- URS Documents for each module
Software Support & Maintenance
Free technical support is provided for the Fusion QbD for 12 months from the date of initial installation. After that, we provide technical support under a Support and Maintenance Agreement (SMA), which is renewable annually. We offer remote support by phone, email and web.
Software updates and upgrades are released on a regular base. Customers with deployments covered under a current SMA are entitled to get those releases without additional costs.
Software Deployment & Integration
There are two editions of the software, that can be provided: Workstation or Network.
Fusion QbD needs to be connected to a CDS system in order to enable data exchange. A particular Offline-Edition is not provided due to the amount of data that needs to be exchanged with the CDS during export and import.
Standalone: FQbD Workstation on CDS Workstation
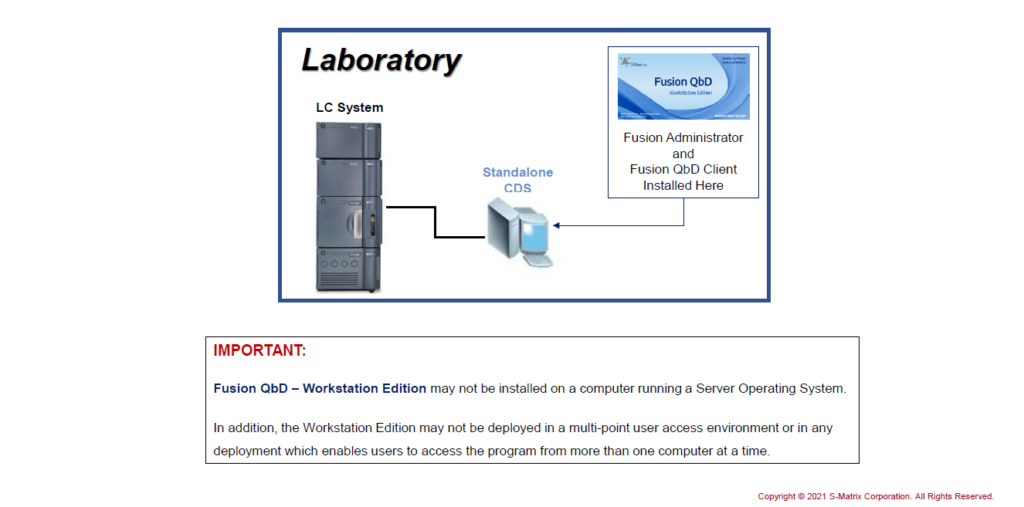
Install the FQbD Workstation Edition on a CDS Standalone Computer.
Hybrid: FQbD Workstation on CDS Network Client
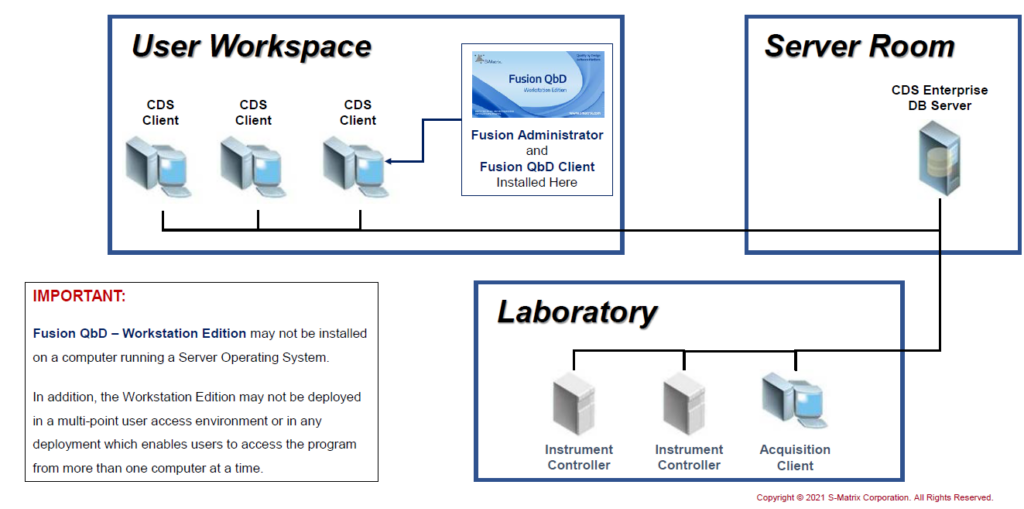
Connect a FQbD Workstation Edition to a CDS Network.
Network: FQbD Network on CDS Network
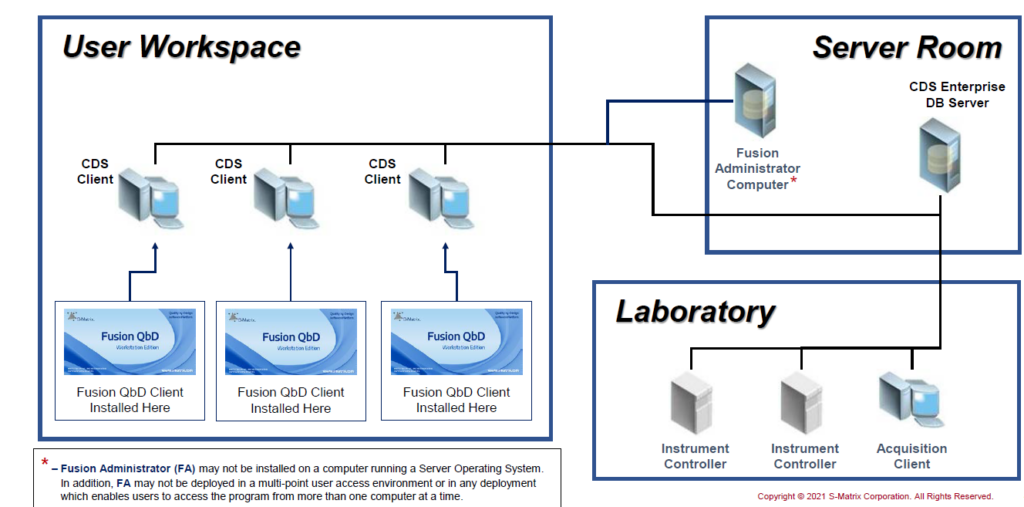
Integrate a FQbD Network Edition into a traditional CDS Network.
Citrix Network: FQbD Network on CDS Terminal Server
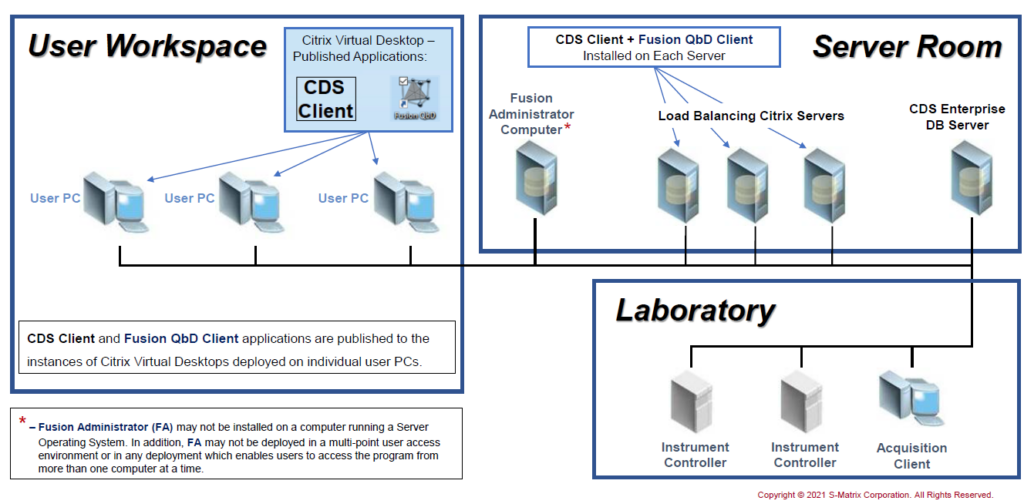
Install the FQbD Network Edition on a CDS Terminal Server.
Fusion QbD is not connected directly to an LC instrument and therefore does not control those devices. Still instrument methods need to be created for the experimental runs and the setup of those methods needs to be matched against the instrument configuration of the LC system. Therefore, those instruments need to be configured in Fusion QbD.
Support for the following LC instruments is currently given:

- Agilent LC Systems, including the Infinity I and II LCs.

- Agilent LC Systems, including the Infinity I and II LCs.
- Dionex LC Systems, including the UltiMate and Vanquish LCs
![]()
- Agilent LC Systems, including the Infinity I and II LCs.
- Waters HPLC, UPLC, and UPC^2 SFC systems.
Fusion Pro - The Pure DoE-Software from S-Matrix
Scientists and engineers usually cannot spend the enormous amount of time and effort to learn the abstract statistical experimental design and data modeling theories which need to be understood to use general statistics software correctly and efficiently, and avoid mistakes common to less experienced users.
With Fusion Pro you can do it yourself without being a statistician!
Fusion Pro is an expert standalone software system which is completely aligned with Quality by Design principles, and designed for scientists and engineers. The software provides a complete suite of Design of Experiments (DOE) technologies, including advanced data modeling and Monte Carlo simulation modeling capabilities, to support your R&D work within a simple workflow based work environment. Fusion Pro is the perfect Design of Experiments (DOE) software for formulation studies, process optimization, and product development, from initial screening through final optimization.
If you only need a DoE software without interface to a chromatography software and without regulatory framework, then Fusion Pro is the product of your choice. Fusion Pro can be considered equivalent to other established statistics programs, but stands out due to its special automation technology. The software is available as stand-alone software but also as a network solution.
Automated Mode selects the most efficient and defensible DOE design for your defined variables and phase of work. User Interactive Mode lets you choose from its extensive design library, but don’t worry, only designs that are statistically valid for your defined variables will be enabled.
Descriptive Statistics Toolset automatically computes analysis ready statistical metrics from data consisting of multiple test repeats per experiment run (e.g. Mean, Variance, Std. Dev., RSD, and %RSD). Time Series Toolset automatically computes analysis ready statistical curve-fit and key time-point metrics (e.g. f1, f2, Weibull, Result at Time Point = X, and Time Point at Result = Y) from data consisting of results run at multiple time points per experiment run (e.g. Dissolution, Synthesis).
Automated Mode automatically derives a statistically defensible model (equation) from your experiment results for each included response. User Interactive Mode gives you full command of the analysis model options and the model building approaches.
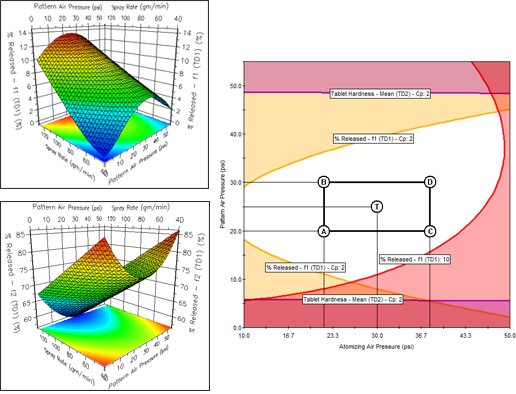
Instantly create 2D Effects Graphs, 3D Response Surface Graphs, and 4D Trellis Graphs. A 4D Trellis is a series of graphs which can show the effects of 4 variables at once on each modeled response or the effects of two critical variables on multiple responses at once.
Mathematically rigorous and correct implementation of Monte Carlo simulation to automatically generate and model key robustness metrics such as Cp and Cpk for all included responses. This powerful capability enables you to characterize and visualize the effects of all study variables on not only mean (average) performance for all included responses, but also their effects on robustness.
You can create a variety of reports containing a single Overlay Graph or a Trellis series of Overlay Graphs depicting your final QbD Design Space. You can also automatically scribe (superimpose) the Proven Acceptable Ranges (PARs) of your study variables on these graphs.
FAQ Section
General Aspects
What is the difference between the Fusion QbD approach for developing analytical methods compared to other tools?
Whereas the technology of other chromatography modelling tools is primarily based on theoretical equations, like the solvophobic one, Fusion uses a real empirical DoE approach. That's also why Fusion can be applied to any chromatography type.
Note: Design of experiments (DoE, german: Statistische Versuchsplanung) is a method for planning experiments that assumes that all experimental results are overlaid by unavoidable random errors. The random errors are taken into account using statistical methods not only during the evaluation, but also during the experimental planning, i.e. before the start of the experimental phase.
As chromatography modelling is based on a statistical approach, how much statistical knowledge is required for working with the software?
Fusion QbD provides DoE designs and statistical modelling to be applied by non-statisticians. The underlying algorithms are developped and patented by S-Matrix and are therefore exclusively provided by Fusion QbD. Note: The automated mode has given evidence being able to replace any user-interactive mode. Still, for statisticians a user-interactive mode is offered in Fusion QbD.
How many replicates for each experimental run are recommended?
As an empirical DoE approach already compensates for the associated random error, no further replicates need to be defined by the operator. The design itsself already includes a certain number of repeats, which are used to calculate the experimental error.
Note: Each observation always underlies a certain random error. Therefore, we would expect that for traditional chromatography modelling tools a mean value of a defined number of replicates is used, as those models do not compensate for the random error associated with each measurement.
Process & Procedure
How many injections are needed for a complete development study?
The length of a design setup depends on several factors such as the number of numeric and categorical study factors, the investigated ranges and the stage of the development phase. Therefore, a general answer cannot be given.
However, as Fusion calculates specific equations and data models for every analytical response from scratch more experiments (i.e. number of different method conditions) are required for screening and optimization experiments compared to an approach where the equations (i.e. a chemical theory) already exist. Still, with the higher number of experiments compensation is already given for the experimental random error. This can only be accounted for by replicate injections using other technologies.
Which pH range do you recommend to start with during a first screening. Would it be sufficient to use one acidic and one basic pH value?
You should not mix basic and acidic pH values during a study, as these are two different chemistry systems. In which of these regions to perform the study should be investigated by a couple of pilot experiments.
You could either select one acidic and one basic pH value and define these buffers as a buffer type variable or you could run several pH values preferably using the OLP mode to investigate, which pH region would work best for your compounds. During screening you should then use pH as a variable with up to 6 pH values and a range of about 1 pH unit.
What accuracy can be expected from the results obtained with Fusion QbD?
This questions probably results from indication such as 70% or 99% of your results will be correct.
Still, this cannot be a way to characterize accuracy in Fusion QbD, as every prediction will have an associated predicition error.
Fusion QbD will indicate the expected mean performance of your results together with a prediction error. The goal should be to keep the predicition error as low as possible. The last is the responsibility of the operator and we provide assistance of how to detect sources for bad predictive value and how to fix this.
Along with the prediction error, there always will be a random error, that is also characterized in Fusion QbD.
From this perspective, you can expect the the reported outcome will always be inside the reported error ranges.
Can predicted results be visualized in Fusion QbD?
Yes, from the calculated models simulation chromatograms can be displayed taking into account elution time, peak shape and peak resolution. In combination with the peak tracking feature peak names can clearly be assigned to the simulated chromatograms.
How does Fusion take different analytical column dimensions into account?
Fusion hasn't implemented a feature to scale a method to different column dimensions and operating conditions that give equivalent chromatographic performance.
However, there are plenty of such tools on the market offered for free by vendors for chromatographic systems and analytical separation columns.
E.g. the Column Calculator from Waters is always shipped together with Empower 3 as a separate app.
Is there a restriction with regard to the number of compounds Fusion is able to manage during an experiment?
The number of compounds actually is not a category Fusion QbD would deal with. We can demonstrate examples with more than 90 compounds.
Still, the more compounds, the more difficult it will be to identify method conditions with satisfactory overall resolution. The multifactorial approach with Fusion QbD will provide a much broader range of possible method conditions compared to any 2D or 3D approach and will let you know, what is possible inside the specified study ranges.
In addition, by extrapolation of the determined response models, promising method conditions outside the actual study space are indicated. Such extrapolation can therefore be considered as proposals that, of course, require verification by a follow-up experiment.
Can gradient steps be defined?
Yes, up to 3 gradient steps can be defined.
However, we recommend trying to avoid a segmented gradient, as with each step a further robustness issue is implemented into the method and a risk for method transfer. Fusion QbD has already proven to develop gradient methods without segmentation and with adequate baseline separation and acceptable run times in multiple customer studies.
Experienced Fusion QbD users know, that the introduction of gradient steps will be a well considered expection, as we can provide many more optimization parameters in a multifactorial approach.
Please also check out our article on this topic:
Can Fusion QbD perform peak tracking?
Fusion QbD 9.9 provides a patented peak tracking technology, that performs hyperaccurate peak tracking by the click on a button. Fusion QbD uses 3D PDA spectral data augmented with standard UV peak results data to automatically identify each peak in each experiment chromatogram. Fusion will also fully utilize 3D mass spectral data for experiments run on LC systems configured with a Waters Acquity single quad mass detector.
Can Fusion QbD handle large molecules where baseline separation is hardly achieved?
As any peak response can be analyzed also not baseline separated peaks can be evaluated using responses such as e.g. retention time difference or p/v ratios.
Are methods developed with Fusion QbD prepared for method transfer?
By defining the permitted and expected maximum variation of all relevant study factors an intended method transfer can already be respected during method development.
We recommend to include an initial isocratic hold time in the pump gradient and study this as a variable method parameter for simulating the dwell volume of different HPLC systems.
Please check the following articles:
How does Fusion QbD determine robustness for the final method?
Fusion QbD determines a multidimensional and multivariate design space, that includes robustness responses. This means robustness is not investigated and characterized after the establishing of an MODR only for single method condtions, but already proven for the entire study region from the obtained data models.
This can be reached by running a Monte Carlo Simulation using the determined models and applying process capability metrics (Cp, Cpk, Cpm, …) before taking the decision for single method conditions or an entire MODR.
Implementation
How does data exchange work between Fusion QbD and a CDS?
Fusion QbD provides automated bidirectional data exchange with Empower, Chromeleon and ChemStation.
Thereby, Fusion QbD does not directly access the database instances in Empower or Chromeleon, but uses the native CDS interfaces for creating ready-to-run sequences and methods during data export and reading out predefined chromatogram results during data import.
For this purpose, Fusion QbD needs to be installed on a CDS client computer.
Can Fusion QbD be used in an IT network?
Yes, Fusion QbD is network capable also in a Citrix environment.
Can Fusion QbD be used in a GxP environment?
Yes, Fusion is prepared for part 11 compliance.
A user management with access groups and different user roles can be defined, all actions can be tracked by an audit trail, e-signature workflows can be configured and data security is also given by the use of encrypted database files and protection of the files that can only be read out in the context of a defined project container.
The software itself is developped alligned with a quality management concept, provides an internal installation qualification and an operational qualification can also be provided by us.
With a main focus on customers in pharmaceutical industry, it is a matter of course for us to be well prepared four our primary target group.
Instrument Support
What instrumentation do you recommend when using Fusion QbD?
Full automation can be provided with an LC system equipped with a quaternary pump and having both column and eluent switching capabilities. With these conditions various mobile and stationary phases including several pH settings can be studied in a single sequence. The online preparation mode for buffer strength, pH, additive and salt concentration eliminates the need to prepare a single eluent solution for each desired condition by building the proportioning required for each experiment run into the instrument methods it automatically constructs.
Which instruments are supported for the control by Fusion QbD?
Fusion QbD offers instrument support for the most commonly used LC systems in pharmaceutical industry and the range of supported instruments increases from year to year. Currently supported instruments are listed here.
FQbD - Supported CDS Systems and LC Platforms - 2022 - S-Matrix
Instrument support means, that the required instrument methods can be created automatically in the respective CDS through the Fusion QbD data exchange functionality.
Can other instruments be used except the listed supported ones?
Yes. You can use FMD for other LC systems than the supported ones, and FPD for any non-LC system ( e.g GC, CE) . With FPD, you can freely compile your design and carry out the data exchange in the same way with the CDS. This already saves a lot of time and provides data security.
However, the exported sequences will still need to be edited by preparing the required instrument methods and adding the necessary equilibration and conditioning steps.
In FPD you will need to to without visualized chromatograms, automated peak tracking and so-called trend responses, i.e. parameters based on the number of peaks.
In addition to Fusion QbD's own modeling technology, the significant advantage compared to other DoE tools is the ability to exchange audited data with a CDS.
Purchase
Can you give any references for success stories with Fusion QbD?
We have collected some show cases in these presentations:
What would be the price for an investment?
The price depends on whether you decide for a standalone or network solution. Beside that there is a license model based on application modules, user and system licenses. Please contact us directly to discuss you individual requirements and situation in order to prepare an individual quote for you.
Playlist

5:52

15:57

5:32

6:54

4:06





